the oil drop test|oil drop experiment diagram : dealer The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment took place in the Ryerson Physical Laboratory . See more Welcome to Redfinger. Using Redfinger requires cellular dat.
{plog:ftitle_list}
WEBPalmeiras e Internacional se enfrentam neste sábado, 11, às 21h, na Arena Barueri, em São Paulo. A partida é válida pela 34ª rodada do Brasileirão. Após derrota contra o .
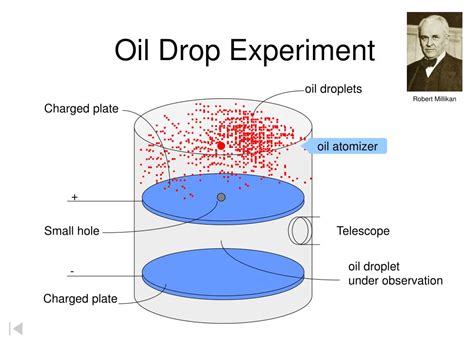
Millikan and Fletcher's experiment involved measuring the force on oil droplets in a glass chamber sandwiched between two electrodes, one above and one below. With the electrical field calculated, they could measure the droplet's charge, the charge on a single electron being (−1.592 × 10−19 C). See moreThe oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). The experiment took place in the Ryerson Physical Laboratory . See more
ApparatusMillikan's and Fletcher's apparatus incorporated a parallel pair of horizontal metal plates. By applying a potential difference across the . See moreIn a commencement address given at the California Institute of Technology (Caltech) in 1974 (and reprinted in Surely You're Joking, Mr. Feynman! in 1985 as well as in The Pleasure of Finding Things Out in 1999), physicist Richard Feynman noted:We have learned a . See more
• Simulation of the oil drop experiment (requires JavaScript)• Thomsen, Marshall, "Good to the Last Drop". Millikan Stories as "Canned" Pedagogy. Eastern Michigan University.• CSR/TSGC Team, "Quark search experiment". . See moreStarting in 1908, while a professor at the University of Chicago, Millikan, with the significant input of Fletcher, the "able assistance of Mr. J. Yinbong Lee", and after improving his . See more
Some controversy was raised by physicist Gerald Holton (1978) who pointed out that Millikan recorded more measurements in his journal than he included in his final results. Holton suggested these data points were omitted from the large set of oil drops measured . See more• Serway, Raymond A.; Faughn, Jerry S. (2006). Holt: Physics. Holt, Rinehart and Winston. ISBN 0-03-073548-3.• Thornton, . See more
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community.
Gain an overview of how Millikan's oil drop experiment is conducted and use the provided formulas to learn how it measures the charge of the electron.Millikan carried out a series of experiments between 1908 and 1917 that allowed him to determine the charge of a single electron, famously known as the oil drop experiment. Millikan sprayed .Robert Millikan’s famous oil drop experiment, reported in August 1913, elegantly measured the fundamental unit of electric charge.Devised by Robert A. Millikan and Harvey Fletcher, the Millikan Oil Drop Experiment is conducted in a chamber and is a method of measuring the electric charge of a single electron. To elaborate, this chamber contains an .
Introduction. The experiment is one of the most fundamental of the experiments in the undergraduate laboratory. The experimental apparatus is patterned after the original .
how to use a universal testing machine compression
oil drop experiment simple diagram
The Millikan oil drop experiment, published in final form in 1913, demonstrated that charge comes in discrete chunks and was a bridge between classical electromagnetism and modern quantum physics.This test involves putting a drop of oil onto a steel surface which is maintained between the boiling points of water and oil. If the oil drop contains water it spits and crackles, hence its name. The crackle test can detect water contamination of less than 0.1 percent, or 1,000 ppm. If a sample fails the crackle test, the actual water content .
The most obvious force is the gravitational pull of the Earth on the droplet, also known as the weight of the droplet. Weight is given by the droplet volume multiplied by the density of the oil (ρ oil) multiplied by the gravitational . The oil drop sign is caused by nail bed parakeratosis and psoriasiform hyperplasia and is a physical finding of psoriasis (1 – 3). Nail psoriasis is common in patients with psoriatic arthritis and can involve the nail matrix (e.g., pitting, trachyonychia, dystrophy, leukonychia) or the nail bed (e.g., onycholysis, subungual hyperkeratosis . Millikan’s oil drop experiment was significant in light of the fact that it assisted with deciding the charge of an electron. This was concluded by estimating the electric field strength expected to prevent progressive oil drops from falling. The oil drop experiment was a scientific experiment that was first performed by Robert Andrew Millikan in the year 1827.
6 A crucial test: the second drop (reading) of 15 March 1912 7 Conclusion: Is closure possible? 1 Introduction Most chemistry and physics textbooks consider the oil drop experiment to be a simple, classic and beautiful experiment, in which Robert A. Millikan (1868-1953) by an exact experimental technique determined the elementary electrical charge.
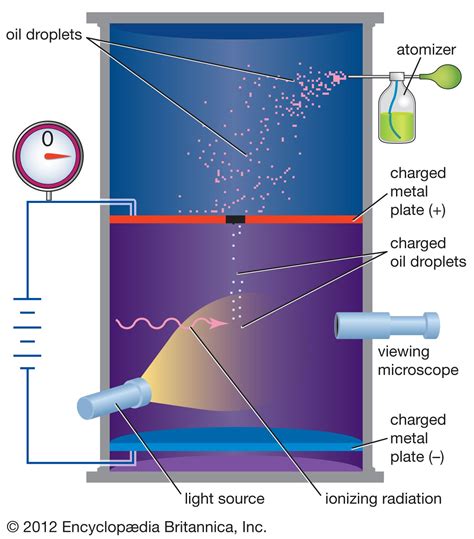
The oil drop experiment that Millikan and Fletcher designed had two chambers. In the upper chamber, an atomizer (like that used in perfume bottles) dispersed a fine mist of micron-sized oil droplets. Individual droplets would fall through a pinhole into the lower chamber, which consisted of two horizontal plates, with one held 16 millimeters .The Millikan oil drop experiment, conducted by Robert Millikan and Harvey Fletcher in 1913, was pivotal in determining the fundamental charge of an electron.Utilizing oil droplets, charged plates, gravity, and X-rays, they were able to measure the electric force and suspend a charged oil droplet between two plates.By adjusting the voltage to counteract gravity, the droplet's . The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron. Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general. Oil Drop Experiment
Spot test. In this test a drop of lube oil is put on the blotter paper and it is then dried for few hours. The dry spot is then compared with the standard spot available which determines the insoluble components in lube oil. 5. Flash point test. This is performed by using Pensky Martin closed cup apparatus which determines the temperature at . Changed oil when I bought it and drop test revealed a very black and thick oil. Changed again 1,000 miles later. Still, very black, but not so thick. Continued to change (using cheap dino) every 2,000 miles afterward and noticed each time, drop test reveals a clearer and cleaner oil. This tells me motor is become cleaner and I can extend OCI on .
This simulation is a simplified version of an experiment done by Robert Milliken in the early 1900s. Hoping to learn more about charge, Milliken sprayed slightly ionized oil droplets into an electric field and made observations of the droplets. When the voltage is zero and the run button is pressed, the drop will fall due to the force of gravity.
The Oil Drop Test involved dropping a known quantity of oil at the beginning of the strip, rolling the strip in the mill, taking a digital photograph of the remaining footprint. This was then down loaded into a computer, scaled and with the use of AutoCAD, the area of . Equipment Set Up for Millikan's Oil Drop Experiment. In Millikan's Oil Drop Experiment oil is sprayed into a chamber before passing between metal plates where the electric and gravitational forces are compared. Condition for Stationary Oil Drops. The charged oil drops fall into a uniform electric field between plates separated by distance d .
oil drop experiment model
Devised by Robert A. Millikan and Harvey Fletcher, the Millikan Oil Drop Experiment is conducted in a chamber and is a method of measuring the electric charge of a single electron. To elaborate, this chamber contains an atomizer, a microscope, a light source, and two parallel metal plates. These metal plates obtain a negative and a positive .The Millikan Oil-Drop Experiment HISTORY The year is 1911, and you are taking a physics course. Your professor is Robert Millikan. Professor Millikan has you and your classmates doing a lab experiment to measure e the magnitude of the charge of an electron, as well as to determine if charge is quantized (in other Oil Drop Experiment. Millikan carried out a series of experiments between 1908 and 1917 that allowed him to determine the charge of a single electron, famously know as the oil drop experiment. He sprayed tiny drops of oil into a chamber. In his first experiment, he simply measured how fast the drops fell under the force of gravity. He could .
In this comprehensive guide, we will delve deep into the world of ASTM Drop Tests, with a particular focus on ASTM D5276 standard. Whether you’re a manufacturer, product designer, or quality assurance professional, .Balance of Forces: Newton’s Law a : radius of drop ρ: density ρ= ρ oil –ρ air v: velocity of oil drop Q: charge of oil drop E: electric field E=V/d V : Voltage across plates η: viscosity of air g : gravitational const. Ö ()) 6 1) dr g dr a ag E g E Fa gz FQ dv F v t E d SK Ö zg 6SK vrag QEE Forces on the oil drop: Fuel dilution blotter test. Thread starter tdi-rick; Start date Dec 22, 2008; Status Not open for further replies. T. tdi-rick. Joined Feb 2, 2004 Messages 1,855 Location Australia. Dec 22, 2008 . (along w/drop in oil pressure cold). D. Doug Hillary. Joined May 30, 2003 Messages 5,118 Location Airlie Beach Australia. Dec 24, 2008 #11 Hi,the test using mineral oil. The drop-collapse test showed the presence of surfac-tant even at low rhamnolipid or surfactin concentrations in the solutions. Moreover, as the surfactant concentra-tion increased, the diameter of the sample drop also increased. This has been reported previously (Bodour & Miller-Maier 1998). The oil surface tension .
Constant Symbol Numerical value Density of oil ρoil 875.3kgm−3 Density of air (at the surface) ρair 1.204kgm−3 Acceleration due to gravity g 9.80ms−2 Dynamic viscosity of air at 1 atm ν 1.827× 10−5Pss The separation of the parallel plates d 6.0mm Table 1: Table of Physical constants for the Millikan experimentMillikan’s Oil Drop Experiment: Measuring the Charge of the Electron. The American scientist Robert Millikan (1868–1953) carried out a series of experiments using electrically charged oil droplets, which allowed him to calculate the charge on a single electron. Millikan created microscopic oil droplets, which could be electrically charged . You want to wait for the paper or card to absorb the oil drop(s) which might take awhile. Once all of the oil has been drawn into the pores of the paper you can begin evaluating the condition of your oil. . Do a search on Google for +blotter +oil +test The other interesting tests are Crackle Test and Oil on Water. R. Russell. Thread starter .
To date, only nine drops have fallen in our famous Pitch Drop experiment. We're home to the famous Pitch Drop experiment, which holds the Guinness World Record for the longest-running laboratory experiment.. The experiment demonstrates the fluidity and high viscosity of pitch, a derivative of tar that is the world's thickest known fluid and was once used for waterproofing .The oil drops picked up static charge and were suspended between two charged plates. Millikan was able to observe the motion of the oil drops with a microscope and found that the drops lined up in a specific way between the plates, based on the number of electric charges they had acquired. Figure \(\PageIndex{2}\): Oil Drop experiment.The drop collapse assay is rapid and easy to carry out, requires no specialized equipment and just a small volume of sample. 36 In addition, it can be performed in microplates. 43 This assay has been applied several times for screening purposes. 2, 9, 36, 44 But it displays a relative low sensitivity since a significant concentration of surface .Hanging drop method is a special type of wet mount where a drop of medium with the organisms is placed on a microscope slide to observe the motility of bacteria. . Remember the high dry objective magnifies a little less than half as much as the oil immersion objective. . Directional purposeful motility is a positive test.
how to use instron universal testing machine

Resultado da 26 de jan. de 2021 · However, the fact that end-to-end encryption is not automatically used on messages sent in Telegram means it’s not as secure as privacy-focused .
the oil drop test|oil drop experiment diagram